Hydrochloric Acid for the processing of a mixed acid solution. (Download)
Mech-Chem Associates engineered, fabricated and installed a distillation system designed to drive off and recover Hydrochloric Acid for the processing of a mixed acid solution.
Our customer faced two needs: First, their source of chemistry was no longer available due to the fact that their supplier was discontinuing production of the main catalyst needed in their chemical process. This meant our customer now had to bring the manufacturing of this chemical in house. Second, their current equipment was incapable of producing the necessary quantities of product, at the required volume and purity, on a reliable and consistent basis. The chemistry begins as a mixture of Hydrochloric Acid (HCl), Water and a second Acid all blended together in a Chemical Mix Tank. The process is heated and mixed in order for the Chlorides to bond with the secondary acid solution. Due to the inefficiency of the reaction, excess amounts of Hydrochloric Acid have to be added to the mix tank in order to ensure the chemical reaction is driven to completion. Once the reaction time is met, the mixed acid solution is pumped into the first Distillation Still. This first still is operated at temperatures up to 245oF. Due to the aggressive nature of the mixed acid all equipment had to be chemical resistant. This particular application used glass lined pipe and vessels, tantalum heat exchangers, graphite gaskets, ceramic valves, and Teflon and tantalum coated instrumentation. The vapor driven off in the first still is cooled by passing it through a heat exchanger that produces a liquid which is 32% by weight Hydrochloric Acid. This acid is stored in a vessel and eventually blended back into the Chemical Mix Tank in order to make the original acid mixture blend. Reclaiming the Hydrochloric Acid at a high concentration and purity reduces the amount of new HCl required to create the initial chemical reaction, resulting in a large economic savings. The next step in the process is to concentrate the remaining liquid in the first still into a crystal. The Facility was using large heated vessels (cookers) to drive away (cook) any residual water and HCl in order to obtain the final product they desired. This process was not only energy intensive but also took a long period of time because the product was only at a concentration of 30% of the remaining solution after the first Distillation Process was completed. Even though the facility had invested in several of the cookers, this inefficient process could not keep up with their production demands. To solve the cooking issue , a second Distillation System was installed to take the concentrate from the first still and bring the concentration of the product from 30% up to 70% before being introduced to the cookers. This process required the second still to operate at temperatures in excess of 300oF. The product in this second still was at 70% before being introduced to the cookers, significantly reducing the time and energy required to produce the final material at the necessary production rate. Additional capital cost no longer had to be outlaid for cookers seeing the Distillation Unit was doing the majority of the work. The acid vapor produced in the second still was 29% HCl and at that concentration the client was able to find a market for selling the recovered HCl. Based on the old method of production, cooking the product from its mixed state to completion with all vapors going to Waste Water Treatment, our customer was able to reuse the HCl produced in the first still. Reusing the HCl in the first still significantly reduced chemistry costs by not having to purchase all new chemistry to start the process in the Chemical Mix Tank. By adding a second still, the customer produced a concentration of HCl that was marketable and had value. These improvements, coupled with the reduction of concentrated waste flowing to Waste Water Treatment and the reduction of energy and time spent cooking the process solution to a concentrate, resulted in the system yielding a cost savings and meeting production needs. Reducing Electronic Waste’s
|
|
The system pictured on the right is a self-contained reactor unit with sliding cover. The reactor system is used for carbon processing operations and metal dissolving, extraction and purification applications.
The pilot system features chemical feed pumps with controllers, containment for acid and chemicals, and fume extraction and ventilation. Materials of construction are welded polypropylene or PVDF with PP, PFA and PVDF piping and shielding. |
|
The pictures to the right and below show a complete automated modular pilot system for the dissolving, processing and purification of various rare earth and precious metals. The pilot system has the following features:
|
|
The picture to the right shows a pilot acid distillation system. The materials of construction are glass components with tantalum heat exchangers assembled with PTFE gaskets. This 10 gallon per hour automated system can be used for the concentration and distillation of various spent or waste acid solutions.
|
Acid Distillation & Fractionation Systems (Download)
Acid evaporation and distillation systems can provide several different functions such as acid separation, concentration and purification. The evaporation and fractionation of mixed acid solutions include the following applications:
- Water removal for acid concentration
- Fractional distillation of mixed acid solutions
- Acid purification for removal of dissolved materials
- Production of high purity electronic grade acids High purity acid production (electronic)
Benefits of Acid Recovery
Of the many options to recover waste or spent acids from manufacturing and metal extraction operations, acid distillation is unique as it can recover and purify the acid while minimizing waste. Acid recovery using distillation can provide a “green technology” with an acceptable Return On Investment. Acid recovery provides the following benefits:
- Good Return On Investment (ROI)
- Reduced Raw Material Costs
- Reduced Waste Disposal Costs
- Reduced Shipping Costs
- Reduced Liability for Transport
- Reduced Storage Requirements
- Up to 95% Waste Reduction
Aircraft Component Manufacturing
Aerospace manufacturers strip the imperfectly-coated and refurbished aircraft components using hydrochloric (HCL) acid so a new metallic coating can be applied. The stripping process produces a contaminated acid solution that requires frequent changing and disposal resulting in higher costs for system shutdown, off-site disposal and new HCL acid purchases. Mech-Chem’s acid recovery systems utilize a distillation column that recovers the spent hydrochloric acid and returns it as a clean distilled acid to the strip line. The system is designed to operate continuously, 24 hours a day, 7 days a week producing a 16% to 20% high purity distilled HCl acid. Continuous operation of the system is achievable using advanced instrumentation and automation technology that is integrated into a PLC with PC interface.
|
Thermal Circulating Systems
Mech-Chem designs, fabricates, and installs a variety of acid distillation and fractionation systems for waste acid recovery and chemical processing applications. The distillation system pictured on the left features a thermal circulating evaporator with a fractionation column that contains a Teflon packing. The materials of construction varies depending on the specific acid evaporation and distillation application. Materials of construction for the distillation units include stainless steel, inconel, Hastelloy, PTFE lined steel, and glass lined steel. The materials of construction for the heat exchangers include stainless steel, inconel, Hastelloy, tantalum, ceramic and carbon graphite. The evaporation and distillation systems are fully instrumented for automated control using a PLC with PC interface.
|
Description of Diffusion Dialysis (Download)
Diffusion dialysis is a membrane separation process. It has been successfully used for many years for the separation and recovery of acids from dissolved metal-bearing solutions. Diffusion is the spontaneous movement of a material from an area of high concentration to an area of lower concentration. Driven by the concentration difference, the movement of material will continue on its own until the concentration difference no longer exists. Dialysis is the separation of molecules due to the differences in the rate of movement of the molecules through a semi-permeable barrier. In the recovery of acids with diffusion dialysis an anion exchange membrane acts as a semi-permeable barrier placed between a flowing water stream and a flowing acid with dissolved metal solution. The anion exchange membrane has fixed positive charges located on its surface. These positive charge locations attract the negatively charged anions in solution that come in close contact with the anion exchange membrane surface.
Anions in the acid solution are attracted to the membrane and they are also driven by the concentration difference to diffuse across the membrane to the water side. Simultaneously, the thermodynamic Law of Electroneutrality (in solution total charge must balance to zero) requires that the transference of every anion be accompanied by the transference of a positive charge. Positively charged ions are strongly inhibited from crossing the positively charged membrane because of the repulsion between like charges. The hydrogen ion, present in the acid solution as H3O+1 ions, or protonated water, is also positively charged, but is able to cross the membrane with very little hindrance. This is due, in part, to the high concentration of hydrogen ion in the acid solution and also, in part, because of the highly associated nature of water, which allows the hydrogen ion to effectively delocalize its charge. The net effect is that the rate of diffusion of acid across the membrane is an order of magnitude greater than that of the dissolved cations. Finally, by causing the flow of the acid solution to be in the opposite direction to the flow of water (counter-current flow), optimal advantage of the necessary concentration gradients can be realized. The results are that the water entering the diffusion dialysis system exists as a metal-depleted recovered acid solution and that the acid solution entering the diffusion dialysis system exists as an acid-depleted dissolved metal-bearing solution.
The Diffusion Dialysis system has two feed tanks, one tank is for water and one is for the acid solution to be processed. After the initial unit start-up and engaging of the automatic control switches on the control panel and level controls in each of the holding tanks, the unit will automatically supply process acid solution and water to the feed tanks. Once the chambers are filled with the acid solution and water, the solutions flow independently by gravity into the membrane stack unit. The acid solution and water flow counter-currently through the membrane stack thus maximizing the concentration gradient. Using the principal of diffusion dialysis, anion exchange membranes segregate acid molecules into a purified zone. In a typical system, 80% to 90% of the sulfuric acid is recovered and 80% to 90% of the dissolved aluminum is removed. The exit ports of the membrane stacks are plumbed to a set of metering pumps, which are used to control the flow of acid solution and water through the System. The exit ports of these metering pumps are plumbed to ½" NPT ball valves to enable hard plumbing of the two streams to their final destination.
The System is a fully modularized design on a single skid. Field installation, by Owner, includes providing acid solution feed and discharge piping, waste discharge piping, compressed air, RO or DI water, and 115v/60Hz/1phase electrical power.
Anions in the acid solution are attracted to the membrane and they are also driven by the concentration difference to diffuse across the membrane to the water side. Simultaneously, the thermodynamic Law of Electroneutrality (in solution total charge must balance to zero) requires that the transference of every anion be accompanied by the transference of a positive charge. Positively charged ions are strongly inhibited from crossing the positively charged membrane because of the repulsion between like charges. The hydrogen ion, present in the acid solution as H3O+1 ions, or protonated water, is also positively charged, but is able to cross the membrane with very little hindrance. This is due, in part, to the high concentration of hydrogen ion in the acid solution and also, in part, because of the highly associated nature of water, which allows the hydrogen ion to effectively delocalize its charge. The net effect is that the rate of diffusion of acid across the membrane is an order of magnitude greater than that of the dissolved cations. Finally, by causing the flow of the acid solution to be in the opposite direction to the flow of water (counter-current flow), optimal advantage of the necessary concentration gradients can be realized. The results are that the water entering the diffusion dialysis system exists as a metal-depleted recovered acid solution and that the acid solution entering the diffusion dialysis system exists as an acid-depleted dissolved metal-bearing solution.
The Diffusion Dialysis system has two feed tanks, one tank is for water and one is for the acid solution to be processed. After the initial unit start-up and engaging of the automatic control switches on the control panel and level controls in each of the holding tanks, the unit will automatically supply process acid solution and water to the feed tanks. Once the chambers are filled with the acid solution and water, the solutions flow independently by gravity into the membrane stack unit. The acid solution and water flow counter-currently through the membrane stack thus maximizing the concentration gradient. Using the principal of diffusion dialysis, anion exchange membranes segregate acid molecules into a purified zone. In a typical system, 80% to 90% of the sulfuric acid is recovered and 80% to 90% of the dissolved aluminum is removed. The exit ports of the membrane stacks are plumbed to a set of metering pumps, which are used to control the flow of acid solution and water through the System. The exit ports of these metering pumps are plumbed to ½" NPT ball valves to enable hard plumbing of the two streams to their final destination.
The System is a fully modularized design on a single skid. Field installation, by Owner, includes providing acid solution feed and discharge piping, waste discharge piping, compressed air, RO or DI water, and 115v/60Hz/1phase electrical power.
Technology, Politics and the Perpetual Motion Machine (Download)
Believe it or not, there is a technology that manufactures a product that combines these seemingly unrelated business and political fields.
The product is manufactured from rare earth metals that are extracted from mining operations using an acid solution. The used waste acid stream which is the byproduct created by stripping the metals from the ore, is recycled for continuous reuse.
In other words “No Waste Stream”, only a product stream that is rich in rare earth metals and a recycled acid stream. The acid is sent back to be reused in the mining operation forming a continuous recycling for the extraction of the rare earth metals.
According to the Law of Conservation of Mass states “for any system matter or energy with mass,
must remain constant over time.” Mining of rare earth metals can apply this law to the separation
and production of the individual metal elements.
By creating recycled acid solutions helps these mining companies minimize chemical usage and waste
streams which is an ongoing issue for this industry.
When you hear the words rare earth metals,
commonly one might instantly think of silver and gold. One could easily overlook the other obvious
metals such as Terbium (used in sonar equipment), Scandium, (manufactured into aerospace
components) or even Thulium (portable X-Ray machines).
The product is manufactured from rare earth metals that are extracted from mining operations using an acid solution. The used waste acid stream which is the byproduct created by stripping the metals from the ore, is recycled for continuous reuse.
In other words “No Waste Stream”, only a product stream that is rich in rare earth metals and a recycled acid stream. The acid is sent back to be reused in the mining operation forming a continuous recycling for the extraction of the rare earth metals.
According to the Law of Conservation of Mass states “for any system matter or energy with mass,
must remain constant over time.” Mining of rare earth metals can apply this law to the separation
and production of the individual metal elements.
By creating recycled acid solutions helps these mining companies minimize chemical usage and waste
streams which is an ongoing issue for this industry.
When you hear the words rare earth metals,
commonly one might instantly think of silver and gold. One could easily overlook the other obvious
metals such as Terbium (used in sonar equipment), Scandium, (manufactured into aerospace
components) or even Thulium (portable X-Ray machines).
Common Use of Rare Earth Metals:
Rare earth metals have a variety of use in the manufacturing industries. These metals range from high
tech equipment, medical equipment, military weapons, and even in common everyday items such as cell phones.
In 2010 China cut their export quota for rare earth metals by 40% causing quite a bit of concern around the globe. As of 2013 China contained 80% of the 17 minerals and announced they were still not meeting their own demand.
However, due to the mobility of American mining industries, along with other companies and entrepreneurs the U.S. was able to re-open several mining operations. The increase production helped to meet the US requirements for these metals.
The irony of the term “rare earth metals” is the fact not that rare. To mine these elements is a combination of labor intensive, complex processes and environmentally hazardous by-products. This
causes concerned companies to look for alternatives to the excessive use of chemistry and having to deal with the environmental impact of this type of mining operation.
Mech-Chem Associates, Inc. has a patented membrane technology called Diffusion Dialysis. This technology separates the metal bearing solution from the acid solution. One side of the membrane produces a metal rich solution that is used for the production of rare earth metals. The other side of the membrane produces the recovered acid solution that is recycled back into the ore leaching operation. This Diffusion Dialysis system keeps the acid solution in a perpetual and sustainable cycle.
tech equipment, medical equipment, military weapons, and even in common everyday items such as cell phones.
In 2010 China cut their export quota for rare earth metals by 40% causing quite a bit of concern around the globe. As of 2013 China contained 80% of the 17 minerals and announced they were still not meeting their own demand.
However, due to the mobility of American mining industries, along with other companies and entrepreneurs the U.S. was able to re-open several mining operations. The increase production helped to meet the US requirements for these metals.
The irony of the term “rare earth metals” is the fact not that rare. To mine these elements is a combination of labor intensive, complex processes and environmentally hazardous by-products. This
causes concerned companies to look for alternatives to the excessive use of chemistry and having to deal with the environmental impact of this type of mining operation.
Mech-Chem Associates, Inc. has a patented membrane technology called Diffusion Dialysis. This technology separates the metal bearing solution from the acid solution. One side of the membrane produces a metal rich solution that is used for the production of rare earth metals. The other side of the membrane produces the recovered acid solution that is recycled back into the ore leaching operation. This Diffusion Dialysis system keeps the acid solution in a perpetual and sustainable cycle.
AREAS OF EXPERTISE (Download)
Mech-Chem is a full service engineering and design firm that specializes in the design of acid and chemical processes and operations. The engineering, design, fabrication and construction capabilities and expertise of our company is in the following applications:
Chemical Processing Systems:
Acid Etching Systems
Acid Pickling Systems
Chemical Milling Systems
Aluminum Etching Systems
Aluminum Anodizing Systems
Metal Processing Operations
Chemical Recovery Systems
Acid Recovery and Purification Systems:
Acid Filtration Systems
Diffusion Dialysis Systems
Acid Distillation Systems
Distillation Fraction Columns
Single Effect Vacuum Evaporators
Vapor Compression Evaporators
Fume Scrubbing Systems:
Push/Pull Ventilation Systems
Vertical Fume Scrubbers
Horizontal Fume Scrubbers
Hoods and Ductwork
Exhaust Blowers
Scrubber PM Services
Wastewater Treatment and Recycling Systems:
Wastewater Treatment Systems
Ion Exchange Resin Systems
Vacuum Evaporation Systems
Carbon Filtration Systems
Zero Discharge Systems
Bulk Storage Facilities and Piping Systems:
Acids/ Alkalis/ Solvents
Bulk Storage Tanks
Pump/Piping Systems
Double Contained Piping Systems
Automated Chemical Feed Systems
Secondary Containments
Truck Containments
Safety and Inventory Control
Process Optimization Services:
Acid Recovery
Waste Recovery
Waste Minimization
Cost Reduction
Pollution Control
EPA & OSHA Compliance
Chemical Processing Systems:
Acid Etching Systems
Acid Pickling Systems
Chemical Milling Systems
Aluminum Etching Systems
Aluminum Anodizing Systems
Metal Processing Operations
Chemical Recovery Systems
Acid Recovery and Purification Systems:
Acid Filtration Systems
Diffusion Dialysis Systems
Acid Distillation Systems
Distillation Fraction Columns
Single Effect Vacuum Evaporators
Vapor Compression Evaporators
Fume Scrubbing Systems:
Push/Pull Ventilation Systems
Vertical Fume Scrubbers
Horizontal Fume Scrubbers
Hoods and Ductwork
Exhaust Blowers
Scrubber PM Services
Wastewater Treatment and Recycling Systems:
Wastewater Treatment Systems
Ion Exchange Resin Systems
Vacuum Evaporation Systems
Carbon Filtration Systems
Zero Discharge Systems
Bulk Storage Facilities and Piping Systems:
Acids/ Alkalis/ Solvents
Bulk Storage Tanks
Pump/Piping Systems
Double Contained Piping Systems
Automated Chemical Feed Systems
Secondary Containments
Truck Containments
Safety and Inventory Control
Process Optimization Services:
Acid Recovery
Waste Recovery
Waste Minimization
Cost Reduction
Pollution Control
EPA & OSHA Compliance
Mech-Chem has an extensive library of over four hundred (400) project designs for systems and facilities for the above applications done by our company's engineering staff. We utilize this knowledge and expertise in the design of each new project done by our company. Mech-Chem engineers and designs user friendly fully automated systems and operations that are monitored and controlled by PLCs with PC interface.
About Mech-Chem Associates, Inc.
Mech-Chem is a full service engineering and design firm that specializes in the engineering, design, fabrication and construction of chemical processes and operations. Mech-Chem was founded in 1984 specializing in acid and chemical corrosion technologies and process design. Over the last 30 years Mech-Chem has grown to include a network of dozens of professionals including an in-house staff of full-time engineers, designers, and PLC programmers.
Our company’s mission is to design, engineer and build industrial processes and systems that not only meet our clients’ production and manufacturing objectives but also meet their environmental, health and safety goals.
Mech-Chem's knowledge of chemical engineering and processing, along with our industrial experience in the design and start-up of these operating systems, gives us the unique ability to design and build fully integrated systems. This expertise allows us to provide our clients with effective processing systems and operations using the best available technology with state of the art PLC/PC automation and controls
Our company’s mission is to design, engineer and build industrial processes and systems that not only meet our clients’ production and manufacturing objectives but also meet their environmental, health and safety goals.
Mech-Chem's knowledge of chemical engineering and processing, along with our industrial experience in the design and start-up of these operating systems, gives us the unique ability to design and build fully integrated systems. This expertise allows us to provide our clients with effective processing systems and operations using the best available technology with state of the art PLC/PC automation and controls
Process Design & Control (Download)
Mech-Chem designs, manufactures, and installs fully automated acid recovery and recycling systems. Systems include diffusion dialysis, vacuum evaporators, distillation systems and filtration/centrifuging equipment. Applications include recovery and recycling of acid solutions. These systems reduce raw material usage, waste treatment, and disposal costs. Mech-Chem interacts with our clients to develop the conceptual design, prepare the detail design, and provide the drawings to permit and construct the facility.
Process Engineering Design
- Process Selection
- Process Flow Diagrams (PFD's)
- Energy and Material Balances
- Equipment and Facility Layouts
- Equipment/ Instrumentation Specifications
- Capital Cost Estimate
Detailed Engineering and Design
- Piping and Instrumentation Diagram (P&ID)
- Structural Drawings
- Mechanical and Piping Drawings
- Electrical/Instrumentation Drawings
- Detailed Equipment Specifications
- Construction Bid Packages
Environmental & Operator Safety
Facilities incorporate state of the art technology to protect the safety of the operators as well as the environment. Trenches are incorporated to keep pipes carrying hazardous materials from being installed overhead. This, along with double walled contained piping and leak detection systems, insure safe operating conditions for everyone in the facility.
Controls & Power Distribution
Mech-Chem also designs, fabricates, and installs the instruments and controls for full PLC automated systems and facilities. Power distribution panels can also be designed, fabricated, and installed for new or refurbished production facilities.
Codes and Regulations
Mech-Chem adheres to the applicable regulations and codes in the engineering, design, and construction of chemical handling and piping systems. For piping systems (including bulk storage, process piping, and secondary containment) these codes include:
- API Codes
- UL Codes
- ASME Codes
- NFPA Fire Protection Codes
- OSHA Safety Regulations
- EPA Environmental Regulations
What is the Most Recycled Product in the U.S.? (download)
According the Scientific American: “The most recycled product is not aluminum cans--only half are recycled. Or even office paper, at more than 70 percent. It's the lead acid batteries from your car. More than 99 percent of such batteries wind up recycled.”
The US Environmental protection Agency states: “Almost any retailer that sells lead-acid batteries collects used batteries for recycling, as required by most state laws.” Reclaimers crush batteries into nickel-sized pieces and separate the plastic components. They send the plastic to a reprocessor for manufacture into new plastic products and deliver purified lead to battery manufacturers and other industries. A typical lead-acid battery contains 60 to 80 percent recycled lead and plastic.
The US Environmental protection Agency states: “Almost any retailer that sells lead-acid batteries collects used batteries for recycling, as required by most state laws.” Reclaimers crush batteries into nickel-sized pieces and separate the plastic components. They send the plastic to a reprocessor for manufacture into new plastic products and deliver purified lead to battery manufacturers and other industries. A typical lead-acid battery contains 60 to 80 percent recycled lead and plastic.
What’s the only component of the Battery not recycled?
“ACID?” The question is “WHY?” when technology exists to also recycle this component of the battery. Automobile batteries contain sulfuric acid, which is commonly referred to as "battery acid". Sulfuric acid is highly corrosive, will burn skin and eyes if contact is made and is poisonous if swallowed. When a battery is cracked, or crushed, during the recycle process, the sulfuric acid is collected. This acid contains several toxic heavy metals such as copper, zinc and lead.
According to AA, the battery recycling industry has several options in dealing with the acid removed from batteries. “It can be neutralized and tested before being released to the environment; or it can be converted to sodium sulfate, a product used in fertilizer, dyes and other products, or reused in new batteries.”
The standard for most of the industries that recycle and/or produce batteries are to simply neutralize the sulfuric acid, and allow the heavy metals to fall out of solution as a solid. The majority of solids are removed from the solution and pressed into a soft cake like mass, which in turn is sent out to landfills for disposal. The remaining liquid is allowed to flow to the local towns waste treatment plant or streams and rivers, depending on which one is the permitted discharge point of the facility handling the waste sulfuric acid.
According to AA, the battery recycling industry has several options in dealing with the acid removed from batteries. “It can be neutralized and tested before being released to the environment; or it can be converted to sodium sulfate, a product used in fertilizer, dyes and other products, or reused in new batteries.”
The standard for most of the industries that recycle and/or produce batteries are to simply neutralize the sulfuric acid, and allow the heavy metals to fall out of solution as a solid. The majority of solids are removed from the solution and pressed into a soft cake like mass, which in turn is sent out to landfills for disposal. The remaining liquid is allowed to flow to the local towns waste treatment plant or streams and rivers, depending on which one is the permitted discharge point of the facility handling the waste sulfuric acid.
Recycling of batteries almost 100% completely recycled?
This magnitude of waste acid does not need to be generated seeing there is technology today to remove the contaminates in the acid and reuse the sulfuric acid in the batteries.
Mech-Chem Associates, Inc. has worked with several recyclers and manufacturers over the last few years. Each using a lab unit to experiment on removal of contaminates in the acid as well as acid strength returned after passing through the dialysis process.
Based on the correspondences we had with these companies, after they had generated enough acid to Beta test batteries, it was noticed by both the sales team, management and engineers that there seemed to be no degradation in the performance or life of the batteries manufactured with the reclaimed acid versus there standard manufacturing practices using new Sulfuric Acid.
Battery Manufacturers stand to save significant money on not having to purchase new sulfuric acid and avoid treating and disposing of all the liquid waste. This would also benefit the environment. The only item which seemed to stand in the way of implementing this technology is the potential concern by the battery manufacturers that the quality of the acid in the batteries would be effected. This however has not happened as the test batteries produced with recycled acid have demonstrated and proven to have the same quality.
How many batteries are recycled each year?
How much acid is contained in those batteries?
I guess “change of ways” sometimes comes slower than “change and advancement of technology”
Mech-Chem Associates, Inc. has worked with several recyclers and manufacturers over the last few years. Each using a lab unit to experiment on removal of contaminates in the acid as well as acid strength returned after passing through the dialysis process.
Based on the correspondences we had with these companies, after they had generated enough acid to Beta test batteries, it was noticed by both the sales team, management and engineers that there seemed to be no degradation in the performance or life of the batteries manufactured with the reclaimed acid versus there standard manufacturing practices using new Sulfuric Acid.
Battery Manufacturers stand to save significant money on not having to purchase new sulfuric acid and avoid treating and disposing of all the liquid waste. This would also benefit the environment. The only item which seemed to stand in the way of implementing this technology is the potential concern by the battery manufacturers that the quality of the acid in the batteries would be effected. This however has not happened as the test batteries produced with recycled acid have demonstrated and proven to have the same quality.
How many batteries are recycled each year?
How much acid is contained in those batteries?
I guess “change of ways” sometimes comes slower than “change and advancement of technology”
Acid Purification Systems (Download)
Acid Purification Systems utilize an easy-to-use, dependable, and economical purification membrane technology known as Diffusion Dialysis.
Mech-Chem manufactures a line of Acid Purifications Systems that utilize the process of diffusion dialysis to remove dissolved metal impurities from used or spent acid solutions and produce a clean, useable acid from what would have otherwise been waste.
Diffusion Dialysis is a very effective technology for the recovery and purification of used, spent, or waste acid solutions that contain low levels of dissolved metals and still contain a large fraction of the acids.
Mech-Chem manufactures a line of Acid Purifications Systems that utilize the process of diffusion dialysis to remove dissolved metal impurities from used or spent acid solutions and produce a clean, useable acid from what would have otherwise been waste.
Diffusion Dialysis is a very effective technology for the recovery and purification of used, spent, or waste acid solutions that contain low levels of dissolved metals and still contain a large fraction of the acids.
Advantages of Acid Recycling
- Reduced acid purchases
- Reduced waste neutralization costs
- Increase acid bath life
- Maintain optimum bath uniformity
- Increase production/reduce downtime
- Reduced hazardous waste disposal costs
- Reduce long-term liability
- Simple, reliable, economical
- Units are self-contained, easily maintained, and require very little floor space
Acid Purification System Overview
In the recovery of acids with diffusion dialysis, an anion exchange membrane acts as a semi-permeable barrier between a flowing water stream and a flowing acid solution that contains the dissolved metals. The anion exchange membrane has fixed positive charges located on its surface. These positive charge locations attract the negatively charged anions in the solution that come in close contact with the anion exchange membrane surface. As a result, the acids in the spent or waste acid solution are attracted to the membrane.
The metal ions which are larger molecules and positively charged are repelled by the positively charged membrane. This allows the acid molecules to diffuse through the membrane at a much faster rate than the dissolved metals. The result is that the water entering a diffusion dialysis system exits as the recovered acid solution containing most of the acid. The spent or waste acid solution entering the diffusion dialysis exits as an acid depleted solution containing most of the dissolved metals. Normal acid recovery is 80% to 90% with removal of 70% to 90% of the dissolved metals.
The metal ions which are larger molecules and positively charged are repelled by the positively charged membrane. This allows the acid molecules to diffuse through the membrane at a much faster rate than the dissolved metals. The result is that the water entering a diffusion dialysis system exits as the recovered acid solution containing most of the acid. The spent or waste acid solution entering the diffusion dialysis exits as an acid depleted solution containing most of the dissolved metals. Normal acid recovery is 80% to 90% with removal of 70% to 90% of the dissolved metals.
Acid Recovery Unit AP– 15 Specifications:
CAPACITY: Up to 15 GPD (56.78 LPD)
LENGTH: 37in (68.6 cm) WIDTH: 27in (94.0 cm) HEIGHT: 66.5in (168.9 cm) WEIGHT: 640lbs (290.3 kilos) TEMP.: 110 F. (43 C.) cooling required above this. POWER: 115 VAC/15 Amp UTILITIES: Water: Up to 20 GPD Air: 30-60 psi FILTERS: 1.0 micron by 10 inch ACID INLET: 0.5in NPT WATER INLET: 0.5in NPT RECLAIM OUTLET: 0.5in NPT METALS OUTLET: 0.5in NPT |
Acid Recovery Unit AP– 30 Specifications:
CAPACITY: Up to 30 GPD (113.56 LPD)
LENGTH: 42in (106.68 cm) WIDTH: 30in (76.2 cm) HEIGHT: 80.5in (204.5 cm) WEIGHT: 760lbs (344.73 kilos) TEMP.: 110 F. (43 C.) cooling required above this. POWER: 115 VAC/15 Amp UTILITIES: Water: Up to 40 GPD Air: 30-60 psi FILTERS: 1.0 micron by 10 inch ACID INLET: 0.5in NPT WATER INLET: 0.5in NPT RECLAIM OUTLET: 0.5in NPT METALS OUTLET: 0.5in NPT |
Acid Recovery Unit AP– 45 Specifications:
CAPACITY: Up to 45 GPD (170.34 LPD)
LENGTH: 42in (106.68 cm) WIDTH: 30in (76.2 cm) HEIGHT: 80.5in (204.5 cm) WEIGHT: 790lbs (358.34 kilos) TEMP.: 110 F. (43 C.) cooling required above this. POWER: 115 VAC/15 Amp UTILITIES: Water: Up to 60 GPD Air: 30-60 psi FILTERS: 1.0 micron by 10 inch ACID INLET: 0.5in NPT WATER INLET: 0.5in NPT RECLAIM OUTLET: 0.5in NPT METALS OUTLET: 0.5in NPT |
Acid Recovery Unit AP– 60 Specifications:
CAPACITY: Up to 60 GPD (227.12 LPD)
LENGTH: 42in (106.68 cm) WIDTH: 30in (76.2 cm) HEIGHT: 80.5in (204.5 cm) WEIGHT: 820lbs (371.95 kilos) TEMP.: 110 F. (43 C.) cooling required above this. POWER: 115 VAC/15 Amp UTILITIES: Water: Up to 80 GPD Air: 30-60 psi FILTERS: 1.0 micron by 10 inch ACID INLET: 0.5in NPT WATER INLET: 0.5in NPT RECLAIM OUTLET: 0.5in NPT METALS OUTLET: 0.5in NPT |
Acid Recovery Unit AP– 150 Specifications:
Feed Module:
CAPACITY: Up to 150 GPD (567.81 LPD) LENGTH: 48in (121.9 cm) WIDTH: 48in (121.9 cm) HEIGHT: 93.5in (237.5 cm) WEIGHT: 1150 lbs (521.63 kilos) TEMP.: 110 F. (43 C.) cooling required above this. POWER: 115 VAC/15 Amp UTILITIES: Water: Up to 300 GPD Air: 30-60 psi FILTERS: 1.0 micron by 10 inch Optional: Duplex Filtration Module Stack Module: CAPACITY: Up to 150 GPD (567.81 LPD) LENGTH: 51.5in (130.8 cm) WIDTH: 36in (91.4 cm) HEIGHT: 42in (106.7 cm) WEIGHT: 720 lbs (326.59 kilos) TEMP.: 110 F. (43 C.) cooling required above this. |
Acid Recovery Unit AP– 300 Specifications:
Feed Module:
CAPACITY: Up to 300 GPD (1135.62 LPD) LENGTH: 48in (121.9 cm) WIDTH: 48in (121.9 cm) HEIGHT: 93.5in (237.5 cm) WEIGHT: 1150 lbs (521.63 kilos) TEMP.: 110 F. (43 C.) cooling required above this. POWER: 115 VAC/15 Amp UTILITIES: Water: Up to 600 GPD Air: 30-60 psi FILTERS: 1.0 micron by 10 inch Optional: Duplex Filtration Module Stack Module: CAPACITY: Up to 300 GPD (1135.62 LPD) LENGTH: 51.5in (130.8 cm) WIDTH: 36in (91.4 cm) HEIGHT: 42in (106.7 cm) WEIGHT: 720 lbs (326.59 kilos) TEMP.: 110 F. (43 C.) cooling required above this. |
Large Scale— Acid Recovery Systems
(Systems larger than 300 gallons per day)
Mech-Chem Associates, Inc. is now offering larger diffusion dialysis membrane stack modules. These modules are the basis for the AP-300 and larger Acid Purification Systems with processing capacities of 300 gallons per day or greater.
The AP-300 and larger acid recovery and purification systems utilize a single feed and control module for the systems. Each system is also equipped with a dual train filtration skid to handle solids removal for the larger industrial applications.
The acid purification system integrates the membrane stack modules with the customer’s feed tanks and process equipment. This results in the acid recovery and purification being an integral part of their production or processing operation.
The diffusion dialysis membrane technology is used for acid recovery applications such as plating baths, anodizing baths, acid pickling, metal finishing applications, and production of aircraft components.
These membrane systems are also finding new applications for recovering and recycling the spent acid streams in mining applications, production of rare earth metals, stainless steel finishing, chemical machining and recovery of electronic components.
Acid recovery and purification has several advantages including reduced acid purchases, hazardous waste disposal and operating costs. In addition it increases the life of the acid solution, maintains optimum bath uniformity , and improves product quality.
The AP-300 and larger acid recovery and purification systems utilize a single feed and control module for the systems. Each system is also equipped with a dual train filtration skid to handle solids removal for the larger industrial applications.
The acid purification system integrates the membrane stack modules with the customer’s feed tanks and process equipment. This results in the acid recovery and purification being an integral part of their production or processing operation.
The diffusion dialysis membrane technology is used for acid recovery applications such as plating baths, anodizing baths, acid pickling, metal finishing applications, and production of aircraft components.
These membrane systems are also finding new applications for recovering and recycling the spent acid streams in mining applications, production of rare earth metals, stainless steel finishing, chemical machining and recovery of electronic components.
Acid recovery and purification has several advantages including reduced acid purchases, hazardous waste disposal and operating costs. In addition it increases the life of the acid solution, maintains optimum bath uniformity , and improves product quality.
Electrodialysis Lab Test Unit (Download)
Mech-Chem offers Electrodialysis lab test units for the testing and verification for the following application:
Applications:
- Removal of Salt Brine from Water.
- Concentration of Metal Salts from Dragout and Rinses for Recycling to Plating Baths.
- Salt Maintenance on Fume Scrubbing Systems
- Electroless Nickel Purifications
Description:
Electrodialysis is an electrically driven ion-exchange process which utilizes alternating sequences of cation exchange membranes and anion exchange membranes to effect the separation and concentration of dissolved salts from various solutions. Selectively permeable ions are transported from one side of a membrane, through the membrane, to the other side of the membrane. These salts are concentrated, collected, and removed from the unit.
With the proper selection and configuration of permselective membranes between a positively charged, inert anode, and a negatively charged cathode, electric deionization is accomplished. Such a configuration of membranes, hydraulic spacers, and electrodes is termed an Electrodialysis stack.
An Electrodialysis system is comprised of an Electrodialysis stack, circulation pumps, solution holding tanks, a power supply, pressure gauges and ancillary controls. Optional controls may also include particulate filters and conductivity control.
With the proper selection and configuration of permselective membranes between a positively charged, inert anode, and a negatively charged cathode, electric deionization is accomplished. Such a configuration of membranes, hydraulic spacers, and electrodes is termed an Electrodialysis stack.
An Electrodialysis system is comprised of an Electrodialysis stack, circulation pumps, solution holding tanks, a power supply, pressure gauges and ancillary controls. Optional controls may also include particulate filters and conductivity control.
Acid Recovery with Diffusion Dialysis (Download)
What is Sulfuric Acid Anodizing?
The Anodizing process produces oxide film on the surface of an aluminum substrate. This acid film produces a hard corrosion and abrasion resistant coating, with excellent wear properties. Various electrolyte solutions can be employed, but the most commonly used is Sulfuric acid.
By controlling the electrolyte and the anodizing conditions, such as temperature, current density and air agitation, one can produce Aluminum coatings with almost any desired property.
Why should I use Diffusion Dialysis with my Sulfuric Acid Anodizing process?
Diffusion Dialysis is ideally suited for the recycling of sulfuric acid anodizing solutions. Diffusion Dialysis provides improved anodize quality, consistent anodized color and consistent anodic thicknesses, cooler and less energy demanding baths, while eliminating production down-time associated with the dumping and remaking of the anodize bath.
The passive, continuous Diffusion Dialysis process enables the anodizer to efficiently remove and control the dissolved aluminum content in the bath while recovering and returning a high percentage of the sulfuric acid back into the process bath. The Diffusion Dialysis process also removes and controls other contaminant buildup in the anodize bath, such as : copper, iron, lead, magnesium, manganese, phosphate, silicon and zinc, while producing a minimum of rejected waste by –product for subsequent treatment and disposal.
The Anodizing process produces oxide film on the surface of an aluminum substrate. This acid film produces a hard corrosion and abrasion resistant coating, with excellent wear properties. Various electrolyte solutions can be employed, but the most commonly used is Sulfuric acid.
By controlling the electrolyte and the anodizing conditions, such as temperature, current density and air agitation, one can produce Aluminum coatings with almost any desired property.
Why should I use Diffusion Dialysis with my Sulfuric Acid Anodizing process?
Diffusion Dialysis is ideally suited for the recycling of sulfuric acid anodizing solutions. Diffusion Dialysis provides improved anodize quality, consistent anodized color and consistent anodic thicknesses, cooler and less energy demanding baths, while eliminating production down-time associated with the dumping and remaking of the anodize bath.
The passive, continuous Diffusion Dialysis process enables the anodizer to efficiently remove and control the dissolved aluminum content in the bath while recovering and returning a high percentage of the sulfuric acid back into the process bath. The Diffusion Dialysis process also removes and controls other contaminant buildup in the anodize bath, such as : copper, iron, lead, magnesium, manganese, phosphate, silicon and zinc, while producing a minimum of rejected waste by –product for subsequent treatment and disposal.
Acid Purification System Model Number
Based upon 24hours per day/ seven days per week of operation. Actual calculation: bath volume divided by calendar days of bath life equals gallons per day required.
Based upon 24hours per day/ seven days per week of operation. Actual calculation: bath volume divided by calendar days of bath life equals gallons per day required.

**Large Scale AP Systems Are Available (Systems greater than 300 Gallons Per Day)**
The Acid Purification Systems manufactured by Mech-Chem Associates, Inc. are tailored to the client’s needs with respect to materials of construction and operating capacity required.
Acid Purification Systems
Mech-Chem’s Acid Purification Systems utilize a dependable and economical anion membrane technology known as Diffusion Dialysis.
Mech-Chem manufactures a line of Acid Purification Systems that utilize the process of diffusion dialysis to remove dissolved metal impurities from used, spent or waste acid solutions. This produces a clean acid solution that can be reused in production operations.
Diffusion Dialysis is a very effective technology for the recovery and purification of used, spent, or waste acid solutions that contain low levels of dissolved metals and still contain a large fraction of the acids. This membrane technology is used for acid recovery applications such as plating baths, anodizing baths, acid pickling and metal finishing applications.
With new emphasis on cost reduction and green technologies, these membrane systems are also finding new applications for recovering and purifying spent or waste acid streams in mining applications and battery acid recycling.
Mech-Chem manufactures a line of Acid Purification Systems that utilize the process of diffusion dialysis to remove dissolved metal impurities from used, spent or waste acid solutions. This produces a clean acid solution that can be reused in production operations.
Diffusion Dialysis is a very effective technology for the recovery and purification of used, spent, or waste acid solutions that contain low levels of dissolved metals and still contain a large fraction of the acids. This membrane technology is used for acid recovery applications such as plating baths, anodizing baths, acid pickling and metal finishing applications.
With new emphasis on cost reduction and green technologies, these membrane systems are also finding new applications for recovering and purifying spent or waste acid streams in mining applications and battery acid recycling.
Acid Purification System Overview
In the recovery of acids with diffusion dialysis, an anion exchange membrane acts as a semi-permeable barrier between a flowing water stream and a flowing acid solution that contains the dissolved metals. The anion exchange membrane has fixed positive charges located on its surface. These positive charge locations attract the negatively charged anions in the solution that come in close contact with the anion exchange membrane surface. As a result, the acids in the spent or waste acid solution are attracted to the membrane.
The metal ions which are larger molecules and positively charged are repelled by the positively charged membrane. This allows the acid molecules to diffuse through the membrane at a much faster rate than the dissolved metals. The result is that the water entering a diffusion dialysis system exits as the recovered acid solution containing most of the acid. The spent or waste acid solution entering the diffusion dialysis exits as an acid depleted solution containing most of the dissolved metals. Normal acid recovery is 80% to 90% with removal of 70% to 90% of the dissolved metals .
The metal ions which are larger molecules and positively charged are repelled by the positively charged membrane. This allows the acid molecules to diffuse through the membrane at a much faster rate than the dissolved metals. The result is that the water entering a diffusion dialysis system exits as the recovered acid solution containing most of the acid. The spent or waste acid solution entering the diffusion dialysis exits as an acid depleted solution containing most of the dissolved metals. Normal acid recovery is 80% to 90% with removal of 70% to 90% of the dissolved metals .
Acid Purification Systems Overview:
Mech-Chem Associates, Inc.’s Acid Purification Systems are simple to operate, dependable, and economical. Our purification systems use an advanced membrane separation technology known as Diffusion Dialysis to separate, recover, and purify strong acids from spent acid solutions contaminated with dissolved metals. An anion exchange membrane serves as a semi-permeable barrier between a flowing stream of water and a flowing stream of process acid. Through this membrane, the processes of both Diffusion and Dialysis occur. These processes are carried out hundreds of times through the numerous internal channels that are contained in the many layers that make up the centrally-located stack of the Acid Purification System.
This technology is capable of recovering acids from concentrated baths that would have been discarded in the past. The purified acid is returned to the process tank for continued use, while a concentrated metal containing aqueous solution is removed for waste treatment. Typically, 80%-90% of the initial process acid is recovered in one pass through the system with 70%-90% removal of dissolved metals from the process acid.
The Acid Purification Systems manufactured by Mech-Chem Associates, Inc. are tailored to client’s needs with respect to materials of construction and operating capacity required. The systems range in size from the AP-15, which processes 15 gallons of acid per day, to the AP-1200, which processes up to 1,200, which processes up to 1,200 gallons of acid per day.
This technology is capable of recovering acids from concentrated baths that would have been discarded in the past. The purified acid is returned to the process tank for continued use, while a concentrated metal containing aqueous solution is removed for waste treatment. Typically, 80%-90% of the initial process acid is recovered in one pass through the system with 70%-90% removal of dissolved metals from the process acid.
The Acid Purification Systems manufactured by Mech-Chem Associates, Inc. are tailored to client’s needs with respect to materials of construction and operating capacity required. The systems range in size from the AP-15, which processes 15 gallons of acid per day, to the AP-1200, which processes up to 1,200, which processes up to 1,200 gallons of acid per day.
- Up to 90% Reduction in New Acid Purchases
- Increase Acid Bath Life/ Decrease Neutralization Costs
- Maintain Bath Uniformity for Optimum Performance
- Increase Production/ Reduce Downtime
- Reduce Hazardous Waste Disposal Costs
AP-L05 Lab Unit
This Acid Purification Lab unit is designed for testing on small volumes of acid solutions, to provide users with an introduction to the technology, and with initial performance data. Evaluation of the results may lead to further pilot scale testing, or to full-scale installation at the facility.
Lab units are available for monthly rental, or may be purchased. The Lab units are designed to process between 1/2 to 1 gallon of acid solution, per 24 hours of operation, depending upon the type of concentrations of acids and dissolved metals in solution.
Lab units are available for monthly rental, or may be purchased. The Lab units are designed to process between 1/2 to 1 gallon of acid solution, per 24 hours of operation, depending upon the type of concentrations of acids and dissolved metals in solution.
Filtration and Centrifuging Systems (Download)
Filtration and Centrifuging Systems are designed to provide mechanical separation and removal of solids and un-dissolved particulates from primary acid solutions, mixed acid solutions, and waste acid solutions. These mechanical separation and removal systems utilize various pieces of equipment including settling tanks, bag filters, cartridge filters and centrifuges. Several systems are illustrated in this brochure.
Many Industrial processes and metal finishing applications require the acid solutions to be disposed of due to the buildup of metal solids in the acid solution. This often occurs before the acids and alkalies in the solution (process bath) has been consumed. Disposal of such waste acid streams is becoming increasingly difficult and expensive.
The continuous recycling and filtering of an acid bath to remove the solids will extend the useful life of the bath. They will produce savings in raw material costs, waste disposal costs, and meeting EPA compliance regulations.
Mech-Chem’s acid and chemical filtration and purification systems are designed to produce a clean, solid free solution with appropriate chemical concentration for use in your manufacturing operations.
The continuous recycling and filtering of an acid bath to remove the solids will extend the useful life of the bath. They will produce savings in raw material costs, waste disposal costs, and meeting EPA compliance regulations.
Mech-Chem’s acid and chemical filtration and purification systems are designed to produce a clean, solid free solution with appropriate chemical concentration for use in your manufacturing operations.
Single Effect Vacuum Evaporators (Download)
Mech-Chem’s single effect vacuum evaporators use conventional climbing film evaporative principles with a horizontal tube arrangement.
The vacuum evaporator is designed to produce a high purity distillate while maximizing volume reduction of the waste, wastewater or waste acid solution being processed.
Mech-Chem fabricates each vacuum evaporator using the appropriate materials of construction for the specified application (i.e. wastewater, salt solution or acid solution). This provides maximum corrosion resistance with a long service life and low maintenance.
The vacuum evaporators are also equipped with NEMA 4x electrical components and PLC Controller. This provides an automated evaporation system that is reliable and easy to operate.
The vacuum evaporator is designed to produce a high purity distillate while maximizing volume reduction of the waste, wastewater or waste acid solution being processed.
Mech-Chem fabricates each vacuum evaporator using the appropriate materials of construction for the specified application (i.e. wastewater, salt solution or acid solution). This provides maximum corrosion resistance with a long service life and low maintenance.
The vacuum evaporators are also equipped with NEMA 4x electrical components and PLC Controller. This provides an automated evaporation system that is reliable and easy to operate.
Wastewater and Rinse Water
- Removal and recovery of heavy metals
- Meet stringent or non-detectable metal limits
- Meet non-detectable mercury limits
- Meet new molybdenum limits
- Used with UF/RO or Ion Exchange to create “Zero Discharge Systems”
- Recover 85%-90% of acids
- Eliminate off-site disposal of waste acid solutions
- Reduce raw material costs
- Eliminate shipping of hazardous chemicals
- Eliminate “cradle to grave” liability
- Chemical milling solutions
- Acid etching solutions
- Metal dissolving acid solutions
- Concentration of plating solutions
- Recycling of plating bath metals
- Concentration of dilute acid solutions
Benefits
Verified Results
Versatile Design
- Operates at low temperatures
- Reduce energy costs
- Vacuum system with no air emissions
- Eliminates potential for vapor leaks
- No external vacuum source
- No environmental permitting required
- Maximum waste/wastewater reduction
- Produces high purity acids
- Safe design (meets OSHA requirements)
Verified Results
- Production of clean, distilled water for recycling
- Production of distilled water with non-detectable metals including mercury and molybdenum
- Recovery of mixed acid solutions
- Production of 50-60wt% high purity nitric acid
- Concentration of plating baths for recycling
- Produces high concentration of waste and reusable by-products
- Recovery of metals from concentrate by-products by relaimers.
Versatile Design
- Mech-Chem’s evaporators are skid mounted modular units with concentrate and distillate tanks.
- Evaporators are equipped with automatic valves and instrumentation for PLC control
- Conventional design for industries with stream, hot water or virtually any waste heat source (all-electric alternative also available)
- Unique vacuum design which provides a totally closed system.
Optimizing Anodizing Baths with Diffusion Dialysis (Download)
By: Daniel E. Bailey
Diffusion dialysis is ideally suited for the recycling of sulfuric acid anodize solutions. Diffusion dialysis provides improved anodize quality, consistent anodic thicknesses, cooler and less energy-demanding baths, while eliminating production downtime associated with the dumping and remaking of the anodize bath. The passive, continuous diffusion dialysis process enables the anodizer to efficiently remove and control the dissolved aluminum content in the bath while recovering and returning a high percentage of the sulfuric acid back into the process bath. The diffusion dialysis process also removes and controls other contaminant build-up in the anodize bath, such as copper, iron, lead, magnesium, manganese, silicon, and zinc, while producing a minimum of rejected waste byproduct for subsequent treatment and disposal.
This article reviews diffusion dialysis technology and relates its benefits to anodizers.
This article reviews diffusion dialysis technology and relates its benefits to anodizers.
WHAT IS DIFFUSION DIALYSIS?
Diffusion dialysis is a membrane separation process. It has been successfully used for many years for the separation and recovery of acids from dissolved metal-bearing solutions. Diffusion is the spontaneous movement of a material from an area of high concentration to an area of lower concentration. Driven by the concentration difference, the movement of material will continue on its own until the concentration difference no longer exists. Dialysis is the separation of molecules due to the differences in the rate of movement of the molecules through a semi-permeable barrier.
In the recovery of acids with diffusion dialysis an anion exchange membrane acts as a semi-permeable barrier placed between a flowing water stream and a flowing acid with dissolved metal solution. The anion exchange membrane has fixed positive charges located on its surface. These positive charge locations attract the negatively charged anions in solution that come in close contact with the anion exchange membrane surface.
In the case of sulfuric acid anodize baths the overwhelmingly predominant anion is the sulfate ion, SO4 . As these sulfate ions in the sulfuric acid anodize solution are attracted to the membrane they are also driven by the concentration difference to diffuse across the membrane to the water side. Simultaneously, the thermodynamic Law of Electroneutrality (in solution total charge must balance to zero) requires that the transference of every sulfate ion, which carries two negative charges, be accompanied by the transference of two positive charges. Positively charged ions, such as Al + 3 or other metal ions, are strongly inhibited from crossing the positively charged membrane because of the repulsion between like charges. The hydrogen ion, present in the acid solution as H3O+1 ions, or protonated water, is also positively charged, but is able to cross the membrane with very little hindrance.
This is due, in part, to the high concentration of hydrogen ion in the acid solution and also, in part, because of the highly associated nature of water, which allows the hydrogen ion to effectively delocalize its charge. The net effect is that the rate of diffusion of sulfuric acid across the membrane is an order of magnitude greater than that of the dissolved aluminum. Finally, by causing the flow of the acid solution to be in the opposite direction to the flow of water (counter-current flow), optimal advantage of the necessary concentration gradients can be realized. The results are that the water entering the diffusion dialysis system exists as a metal-depleted recovered acid solution and that the acid solution entering the diffusion dialysis system exists as an acid-depleted dissolved metal-bearing solution.
In the recovery of acids with diffusion dialysis an anion exchange membrane acts as a semi-permeable barrier placed between a flowing water stream and a flowing acid with dissolved metal solution. The anion exchange membrane has fixed positive charges located on its surface. These positive charge locations attract the negatively charged anions in solution that come in close contact with the anion exchange membrane surface.
In the case of sulfuric acid anodize baths the overwhelmingly predominant anion is the sulfate ion, SO4 . As these sulfate ions in the sulfuric acid anodize solution are attracted to the membrane they are also driven by the concentration difference to diffuse across the membrane to the water side. Simultaneously, the thermodynamic Law of Electroneutrality (in solution total charge must balance to zero) requires that the transference of every sulfate ion, which carries two negative charges, be accompanied by the transference of two positive charges. Positively charged ions, such as Al + 3 or other metal ions, are strongly inhibited from crossing the positively charged membrane because of the repulsion between like charges. The hydrogen ion, present in the acid solution as H3O+1 ions, or protonated water, is also positively charged, but is able to cross the membrane with very little hindrance.
This is due, in part, to the high concentration of hydrogen ion in the acid solution and also, in part, because of the highly associated nature of water, which allows the hydrogen ion to effectively delocalize its charge. The net effect is that the rate of diffusion of sulfuric acid across the membrane is an order of magnitude greater than that of the dissolved aluminum. Finally, by causing the flow of the acid solution to be in the opposite direction to the flow of water (counter-current flow), optimal advantage of the necessary concentration gradients can be realized. The results are that the water entering the diffusion dialysis system exists as a metal-depleted recovered acid solution and that the acid solution entering the diffusion dialysis system exists as an acid-depleted dissolved metal-bearing solution.
APPLIED DIFFUSION DIALYSIS
The standard processing rate for diffusion dialysis systems is a liter per hour per square meter (approximately 0.025 gal/hr/ft2 ) of available anion exchange membrane surface area. To obtain the necessary membrane area that is required to process large volumes, the membranes are stacked between gasketed hydraulic flow spacers. These membrane stacks are usually standardized over a range of differing processing capacities.
Figure 1 depicts a typical, automatically operated acid-recycling configuration. The acid-recycling system has two liquid chambers at the top of the unit: one chamber is for water and the other is for the acid to be processed. A dual set of level controls is located in each chamber. As the acid level drops in the chamber the primary level controller will energize a pump located on the base of the system. Acid solution will be drawn into this pump and then sent through a filter and into the acid holding chamber on top of the module.
Once the acid-holding chamber has been refilled the primary level controller will shut off the pump. Should the primary level controller fail for some reason a secondary level controller will shut off power to the system at emergency-high or emergency-low level and an audible alarm will sound. A similar dual arrangement is present in the water-holding chamber. Instead of a pump the primary level controller is tied into a solenoid valve, which is plumbed to the water feed line.
Once the water and acid solutions are in the holding chambers on the unit they flow independently by gravity into the membrane stack on the base of the unit (see Fig. 2). The acid and water solutions flow counter-currently through the membrane stack, thus maximizing usage of the concentration gradients. Using the principles of diffusion dialysis, anion exchange membranes segregate acid molecules into a purified zone. Typically, 80 to 95% of the acid is recovered with 80 to 95% of the metals removed.
The exit ports of the membrane stack are plumbed to a set of metering pumps. Except during the automatic refilling of the system these metering pumps are the only moving components on the entire system. The metering pumps are used to control the solution flow rates. The exit ports of these metering pumps are plumbed either back into the acid process tank, for the recovered acid stream, or in the case of the metal-rich, acid-depleted waste, plumbed to final treatment. The acid-recycling system is a fully modularized unit. For installation the pump on the acid-recycling unit is plumbed to the working anodize tank(s) and a solenoid valve on the unit is plumbed to a pressurized water source.
Figure 1 depicts a typical, automatically operated acid-recycling configuration. The acid-recycling system has two liquid chambers at the top of the unit: one chamber is for water and the other is for the acid to be processed. A dual set of level controls is located in each chamber. As the acid level drops in the chamber the primary level controller will energize a pump located on the base of the system. Acid solution will be drawn into this pump and then sent through a filter and into the acid holding chamber on top of the module.
Once the acid-holding chamber has been refilled the primary level controller will shut off the pump. Should the primary level controller fail for some reason a secondary level controller will shut off power to the system at emergency-high or emergency-low level and an audible alarm will sound. A similar dual arrangement is present in the water-holding chamber. Instead of a pump the primary level controller is tied into a solenoid valve, which is plumbed to the water feed line.
Once the water and acid solutions are in the holding chambers on the unit they flow independently by gravity into the membrane stack on the base of the unit (see Fig. 2). The acid and water solutions flow counter-currently through the membrane stack, thus maximizing usage of the concentration gradients. Using the principles of diffusion dialysis, anion exchange membranes segregate acid molecules into a purified zone. Typically, 80 to 95% of the acid is recovered with 80 to 95% of the metals removed.
The exit ports of the membrane stack are plumbed to a set of metering pumps. Except during the automatic refilling of the system these metering pumps are the only moving components on the entire system. The metering pumps are used to control the solution flow rates. The exit ports of these metering pumps are plumbed either back into the acid process tank, for the recovered acid stream, or in the case of the metal-rich, acid-depleted waste, plumbed to final treatment. The acid-recycling system is a fully modularized unit. For installation the pump on the acid-recycling unit is plumbed to the working anodize tank(s) and a solenoid valve on the unit is plumbed to a pressurized water source.
IMPLEMENTING ACID RECYCLING
In 1995 three 10 gpd diffusion dialysis systems were installed onto three 1,000-gal sulfuric acid anodize tanks at an anodizing job shop located in Santa Clara, Calif., for control of aluminum and contaminant build-up and recovery of sulfuric acid. Quality control through consistent anodize bath purity was a major motivating force in the implementation of these units.
To prove its effectiveness in removing metallic contaminants and in producing workable concentrations of acid, pilot studies were performed on the anodizing solutions. The pilot studies showed excellent results in removing metallic contaminants as well as generating a recovered acid permeate of sufficient concentration for reuse. The acid depleted fraction following dialysis produced a solution, which was rich in metal and weak in acid concentration.
The sizing of the diffusion dialysis system was based upon the volume of spent anodizing solution that was previously produced, the rate of this production, and the efficiency of the diffusion dialysis process. A useful "rule of thumb" requires that, at a minimum, the volume of spent acid that was previously discarded be recycled once through the diffusion dialysis unit over the same period of time that it took to generate the spent acid.
The three 10-gpd acid-recycling systems were installed directly onto the anodizing process tanks. Additions of virgin acid are made to replenish depleted volumes due to consumption, dragout, exhaust escape, and (he minor amounts lost in the dialysis process.
Table I is representative of the diffusion dialysis performance results of this installation. Recycling efficiencies were calculated by comparing the recycled acid concentrations to the i n iti a l anodize bath concentrations.
In 1995 an anodizing job shop located in Pawtuckct, R.I., installed a 20- gpd diffusion dialysis system onto a 1,000-gal sulfuric acid anodize tank for control of a l umi n um and improved quality control. In 1996 another 20-gpd diffusion dialysis system was installed onto a 1,000-gal sulfuric acid hardcoat tank that contained glycerin as an additive. It was found that about two-thirds of the glycerin was also recovered with the reclaimed sulfuric acid. These anodizing baths have not been dumped since.
To prove its effectiveness in removing metallic contaminants and in producing workable concentrations of acid, pilot studies were performed on the anodizing solutions. The pilot studies showed excellent results in removing metallic contaminants as well as generating a recovered acid permeate of sufficient concentration for reuse. The acid depleted fraction following dialysis produced a solution, which was rich in metal and weak in acid concentration.
The sizing of the diffusion dialysis system was based upon the volume of spent anodizing solution that was previously produced, the rate of this production, and the efficiency of the diffusion dialysis process. A useful "rule of thumb" requires that, at a minimum, the volume of spent acid that was previously discarded be recycled once through the diffusion dialysis unit over the same period of time that it took to generate the spent acid.
The three 10-gpd acid-recycling systems were installed directly onto the anodizing process tanks. Additions of virgin acid are made to replenish depleted volumes due to consumption, dragout, exhaust escape, and (he minor amounts lost in the dialysis process.
Table I is representative of the diffusion dialysis performance results of this installation. Recycling efficiencies were calculated by comparing the recycled acid concentrations to the i n iti a l anodize bath concentrations.
In 1995 an anodizing job shop located in Pawtuckct, R.I., installed a 20- gpd diffusion dialysis system onto a 1,000-gal sulfuric acid anodize tank for control of a l umi n um and improved quality control. In 1996 another 20-gpd diffusion dialysis system was installed onto a 1,000-gal sulfuric acid hardcoat tank that contained glycerin as an additive. It was found that about two-thirds of the glycerin was also recovered with the reclaimed sulfuric acid. These anodizing baths have not been dumped since.
COMPARISON OF DIFFUSION DIALYSIS TO RESIN SORPTION TECHNOLOGY
Resin sorption technologies have often been successfully utilized for the recycling of aluminum anodizing baths. This technology relies on the sorption of acid molecules on an ion exchange resin bed. The process works by pumping contaminated acid into the bottom of the resin bed. Acid is sorbed by the resin particles and the partially deacidified salt solution is collected from the top of the bed. Water is then pumped into the top of the bed, desorbing the acid from the resin and the recovered acid product is collected from the bottom of the bed. The above cycle is continuously repeated by alternately opening and closing a series of valves.
Acid recovery efficiency via resin sorption can vary from 70 to 95% per pass; however, metal removal rates per pass can be as low as 25%. One reason for this low aluminum removal efficiency is due to the entrapment of process solution that is still contaminated with aluminum in the resin bed column. This entrapment hinders overall recycling efficiency because it requires multiple passes to achieve sufficient metal removal. For example if a bath is maintained at 6 g/L aluminum and the bath normally builds up to a tolerable limit of 12 g/L in 10 days then the system must remove a mass of aluminum equivalent to 6 g/L times the entire volume of the bath in the same 10 days. If the system has an aluminum removal efficiency of 25% per pass it must process the entire vol-ume of the bath 4 times in 10 days. Assuming that 90% of the acid is recovered in each pass and that the acid concentration in the bath is maintained at 15% by weight, 1.5% by weight of the acid is lost in each pass. After 4 passes a total of 6% by weight of the acid is lost.
By contrast, diffusion dialysis systems will typically remove 90% or greater of the aluminum per single pass while recovering 90% of the acid. In the hypothetical case described above -a diffusion dialysis system will require 1.1 passes (6 g/L H- [0.9 X 6 g/L]) to remove 6 g/L of aluminum. After 1.1 passes at 90% acid recovery a total of 1.7% by weight of acid will be lost. The effective net efficiency of acid recovery is, therefore, only 89%. Thus, significantly less waste byproduct is produced, typically one-half to one fifth as much. When assessing the benefits of any recycling technology it is important to closely look at the volume and content of the waste byproducts produced and balance this with the volume and content of the products recovered.
With the advent of significantly more durable ion exchange membranes in recent years, the life expectancy of the ion exchange membranes utilized in sulfuric acid recovery is between 10 to 20 years. Typical ion exchange resin life in acid sorption systems varies between 2 to 10 years. Both technologies require very good prefiltration of the process solution prior to introduction into the recovery units.
Acid recovery efficiency via resin sorption can vary from 70 to 95% per pass; however, metal removal rates per pass can be as low as 25%. One reason for this low aluminum removal efficiency is due to the entrapment of process solution that is still contaminated with aluminum in the resin bed column. This entrapment hinders overall recycling efficiency because it requires multiple passes to achieve sufficient metal removal. For example if a bath is maintained at 6 g/L aluminum and the bath normally builds up to a tolerable limit of 12 g/L in 10 days then the system must remove a mass of aluminum equivalent to 6 g/L times the entire volume of the bath in the same 10 days. If the system has an aluminum removal efficiency of 25% per pass it must process the entire vol-ume of the bath 4 times in 10 days. Assuming that 90% of the acid is recovered in each pass and that the acid concentration in the bath is maintained at 15% by weight, 1.5% by weight of the acid is lost in each pass. After 4 passes a total of 6% by weight of the acid is lost.
By contrast, diffusion dialysis systems will typically remove 90% or greater of the aluminum per single pass while recovering 90% of the acid. In the hypothetical case described above -a diffusion dialysis system will require 1.1 passes (6 g/L H- [0.9 X 6 g/L]) to remove 6 g/L of aluminum. After 1.1 passes at 90% acid recovery a total of 1.7% by weight of acid will be lost. The effective net efficiency of acid recovery is, therefore, only 89%. Thus, significantly less waste byproduct is produced, typically one-half to one fifth as much. When assessing the benefits of any recycling technology it is important to closely look at the volume and content of the waste byproducts produced and balance this with the volume and content of the products recovered.
With the advent of significantly more durable ion exchange membranes in recent years, the life expectancy of the ion exchange membranes utilized in sulfuric acid recovery is between 10 to 20 years. Typical ion exchange resin life in acid sorption systems varies between 2 to 10 years. Both technologies require very good prefiltration of the process solution prior to introduction into the recovery units.
JUSTIFICATION AND BENEFITS
At anodizing job shops and captive shops across the U.S. diffusion dialysis users report improved anodize quality and reduced rework, often with reduced processing times. Additionally, dialyzed anodize baths tend to run cooler, using less energy, and thus cost less to operate. The following is a summary of benefits being derived from the implementation of acid recycling utilizing diffusion dialysis:
- Savings from reduced or eliminated disposal costs and reduced acid purchases
- Elimination of production downtime associated with the dumping and recharging of acid baths
- Minimization of direct operator contact with dangerous chemicals—reduced operator exposure
- Fully automatic operation, 24 hours per day, 7 days per week, with very minimal operating costs
- Improved process control with consistent anodic thicknesses improves quality and minimizes waste
Summary
Diffusion dialysis for acid recycling has many benefits. It reduces acid purchases and eliminates or lowers neutralization or hazardous waste hauling costs and the related liability. Toxic chemical use is reduced and the required reporting and handling of hazardous materials and associated labor is greatly reduced. Consistent bath strength yields greater product uniformity and better quality with lower operating costs. Diffusion dialysis can dramatically improve a facility's quality and economic performance.
Mech-Chem Associates, Inc.
Chemical Engineering:
Design, Engineering, Fabrication & Installation
Archives
May 2017
August 2016
April 2016
May 2015
November 2014
September 2014
January 2014
August 2013
February 2013
December 2012
July 2012
May 2012
March 2012
March 2011
November 1995



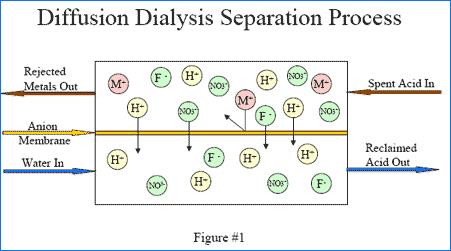
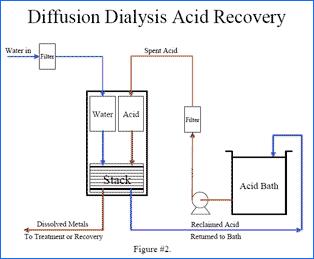
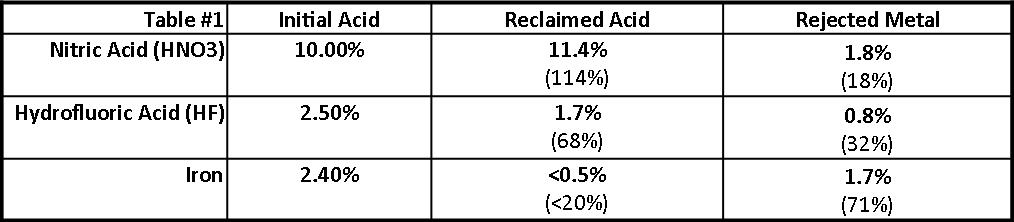








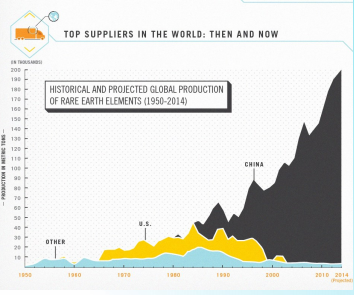



















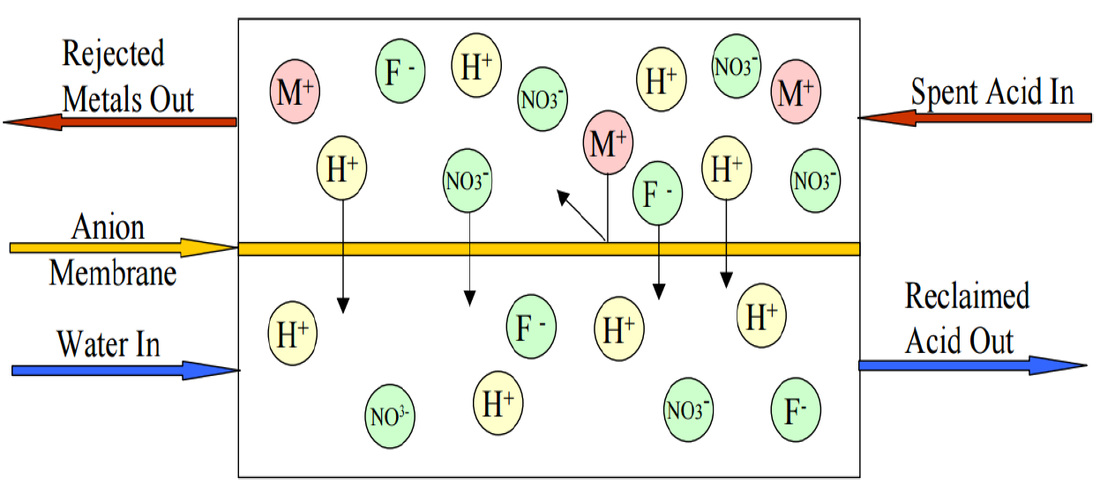





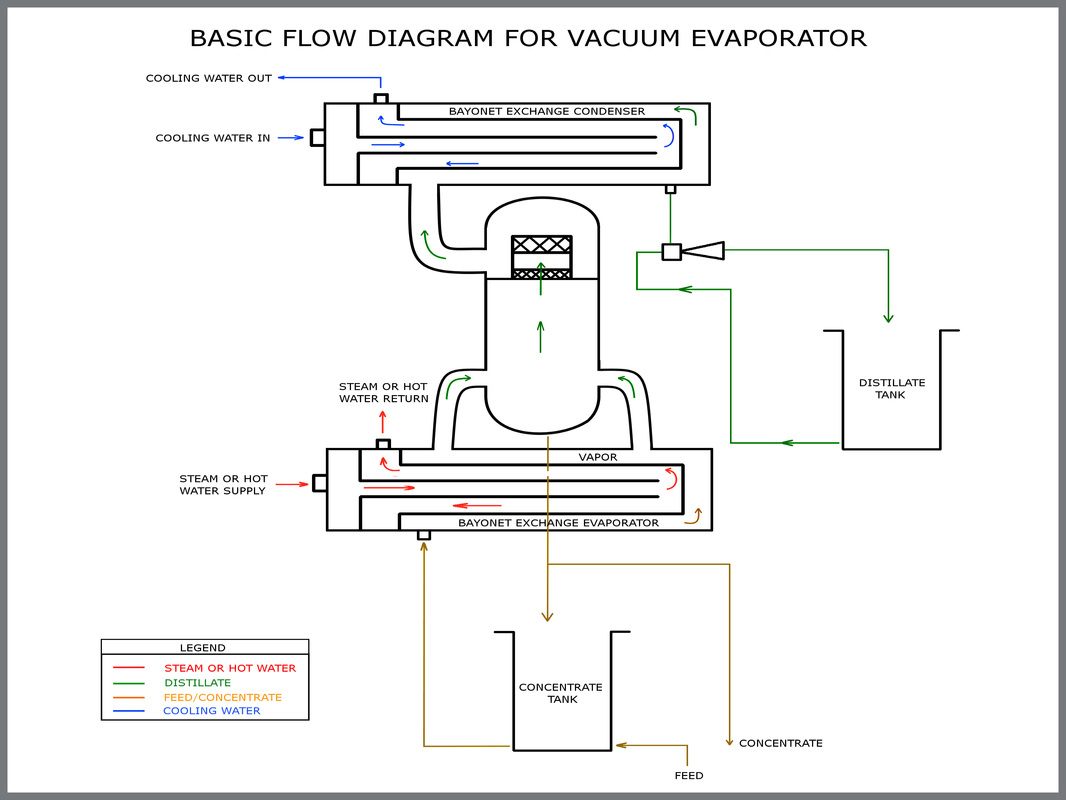
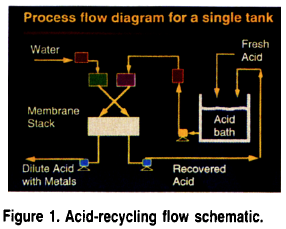
 RSS Feed
RSS Feed
